


Anyway, okay, so when we add these together, that still gives us 4 electron domains and that's what the question is telling you is that hay they have the same number of electron domains. This VESPR procedure is summarized as follows: Draw the Lewis electron structure of the molecule or polyatomic ion. So it has that 1 lone pair of electrons here on the top of the peak, a rot on the top. So as we look at the phosphorus, we see that it has 3 atoms bonded to it and it has 1 lone pair. Predict the geometries of the following species using the VSEPR method: (a) PCl3, (b) CHCl3, (c) SiH4, (d)TeCl4. L 3 point: so we have a phosphorus phosphorus, has 5 valence electrons of its own, so it's going to have a lone pair and then it will have single bonds to each of 3 chlorine's and each of the chlorine has a flacket but we're interested in our Central atom here, the phosphorus. So therefore, the molecular geometry is also a tetrahedron.
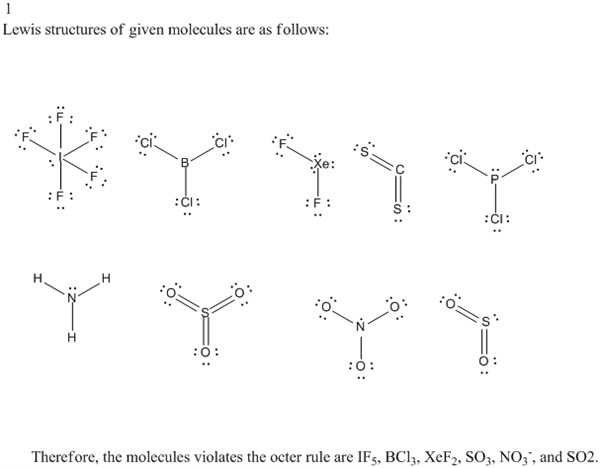
2) Always the most electronegative element in the middle: P. Then there are actually atoms attached to all 4 of these electron domains. 5 steps: 1) Find total number of valence: P 5 and Cl3 21 T ot 26. So we have an electron domain geometry, which is a tetrahedral in this molecule. That means the electrons arrange themselves in a tetrahedral shape.

Lone pairs on the carbon, that's all we're looking as the central atom, so adding these 2 together is how we get 4 electron domains when there are 4 electron domains. So as we look at our central atom carbon here, we see that it has 4 atoms bonded in 0. Ccl 4 means carbon is bonded with a single bond to each chlorine, and each chlorine then has 3 lone pairs to give each chlorine an up tete. Hi there to answer this question, i want to start off by sketching the lewis structure, or at least the structural formula, for each of these. The nonbonding electron domain will cause a slight distortion in the geometry. PCl3: There are three bonding electron domains and one nonbonding electron domain. Thus, both CCl4 and PCl3 have the same electron-domain geometry, which is $\boxed$. View Answer Provide the electron pair geometry and the molecular geometry for the given. So, there are 4 electron domains around the central atom. Provide the electron pair geometry and the molecular geometry for the following compound. PCl3: Phosphorus (P) is the central atom, and it forms three single bonds with three Chlorine (Cl) atoms.Īdditionally, there is one lone pair of electrons on the Phosphorus atom. So, there are 4 electron domains around the central atom. Electron domains include both bonding and nonbonding electron pairs.ĬCl4: Carbon (C) is the central atom, and it forms four single bonds with four Chlorine (Cl) atoms. With fewer 90° LP–BP repulsions, we can predict that the structure with the lone pair of electrons in the equatorial position is more stable than the one with the lone pair in the axial position.(a) To determine the electron-domain geometry of CCl4 and PCl3, we need to consider the number of electron domains around the central atom. If we place it in the axial position, we have two 90° LP–BP repulsions at 90°. However, because the axial and equatorial positions are not chemically equivalent, where do we place the lone pair? If we place the lone pair in the equatorial position, we have three LP–BP repulsions at 90°. We designate SF 4 as AX 4E it has a total of five electron pairs.


 0 kommentar(er)
0 kommentar(er)
